guest post:
Valacyclovir associated Neurotoxicity - Highly Preventable Adverse Drug Reactions in Chronic Kidney Disease
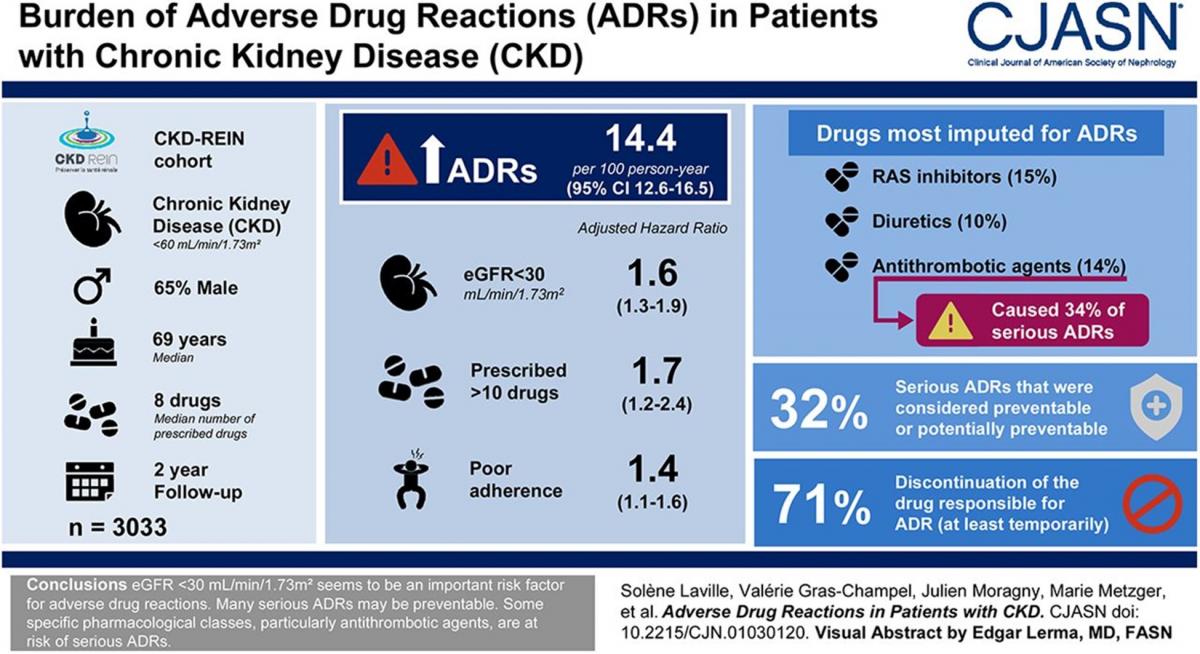
An estimated 4 million Canadians are affected by chronic kidney disease (CKD).1 Patients with chronic kidney disease (CKD) are on a median of 8 drugs and are at high risk of experiencing adverse drug reactions (ADRs). The Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) found a rate of 14.4 ADRs per 100 person years with serious ADRs occurring at a rate of 2.7 per 100 person years. The authors also found that 32% of the serious ADRs were considered preventable. Patients with an eGFR of less than 30mL/min/1.73m2 are more likely to experience ADRs, with a hazard ratio of 1.6.2
Valacyclovir is widely used for the treatment of varicella-zoster virus; however, one notable adverse event is neurotoxicity. This commonly presents as confusion, drowsiness, stupor and, in more severe cases, coma, and often occurs within 1 to 5 days from treatment initiation. A recent systematic review examined reported cases of acyclovir and valacyclovir associated neurotoxicity and found that 83.3% of the cases had documented history of renal impairment and 57.1% noted end-stage renal disease (ESRD). In 59.7% of cases, the dose was higher than recommended, reflecting inappropriate renal dose adjustment.3
Valacyclovir is a pro-drug of acyclovir and gets rapidly converted to acyclovir via first-pass effect. There is very minimal further hepatic metabolism of acyclovir and 89% is ultimately excreted via the urine. Due to it being primarily cleared by the kidney, there is an expected increase in elimination half-life in patients with renal impairment: 2.5 to 3.3 hours in normal renal function, compared with 14 to 20 hours in ESRD.4 Additionally, both acyclovir and valacyclovir have a low molecular weight and limited protein binding, therefore they are extensively removed by dialysis.5,6 Taking all these pharmacokinetic considerations into account, there is significant renal dose adjustment indicated in patients with CKD. For example, the standard treatment dose of valacyclovir for herpes zoster (shingles) is 1 g orally three times daily. In patients with ESRD, this dose should be reduced to 500 mg orally once daily – one sixth of the usual dose. In hemodialysis, valacyclovir should be administered after dialysis on dialysis days.4
With proper dose adjustment, valacyclovir associated neurotoxicity in advanced CKD is highly preventable and can avoid distressing symptoms and hospital admissions. While ensuring a safe medication regimen is a shared responsibility, that includes the original prescriber, the community pharmacist’s expertise in pharmacokinetics serves an important role in potentially intervening for appropriate dose adjustments. Pharmacists in British Columbia can enroll to obtain access to CareConnect, an electronic health record, which provides online access to lab results and other clinical information.7 A quick bloodwork review, followed by a dose adjustment as allowed by the pharmacists’ authority to adapt a prescription under Section 12 of Professional Practice Policy-58 (PPP-58) should be encouraged for all drugs that are predominantly renally eliminated with high risk for adverse events.8
About the Authors:
Nina Bredenkamp, BSc.(Pharm), ACPR
Clinical Pharmacist, Fraser Health Renal Program
Ada Chiu, BSc.(Pharm), ACPR, PharmD, BCPS
Clinical Pharmacy Specialist, Fraser Health Renal Program
Claudia Ho, BSc.(Pharm), ACPR, PharmD, BCPS
Clinical Pharmacy Specialist, Fraser Health Renal Program
References:
- Manns B, McKenzie SQ, Au F, Gignac PM, Geller LI. The financial impact of advanced kidney disease on Canada Pension Plan and private disability insurance costs. Can J Kidney Heal Dis. 2017;4. doi:10.1177/2054358117703986
- Laville SM, Gras-Champel V, Moragny J, et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol. 2020;15(8):1090-1102. doi:10.2215/CJN.01030120
- Brandariz-Nuñez MPharm D, Correas-Sanahuja BPharm M, Maya-Gallego S, Martín Herranz MPharm I. Neurotoxicity associated with acyclovir and valacyclovir: A systematic review of cases. J Clin Pharm Ther. 2021;46:918-926. doi:10.1111/jcpt.13464
- UpToDate LexiDrug. Valacyclovir. Lexi-Drugs. Published March 19, 2025. Accessed March 25, 2025. https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7849?cesid...
- Valtrex (valacyclovir) [product monograph]. Mississauga, Ontario: GlaxoSmithKline Inc.; 2022.
- Apo-Acyclovir (acyclovir) [product monograph]. Toronto, Ontario: Apotext Inc.; 2014.
- College of Pharmacists of British Columbia. Guest Post: 3 Reasons Why CareConnect Improves Patient Care in a Pharmacy Setting. Published July 27, 2021. Accessed March 25, 2025. https://www.bcpharmacists.org/readlinks/guest-post-3-reasons-why-carecon...
- College of Pharmacists of British Columbia. Professional Practice Policy 58: Adapting a Prescription.; 2024. Accessed March 25, 2025. https://library.bcpharmacists.org/6_Resources/6-2_PPP/5003-PGP-PPP58.pdf