Board Highlights - September 24, 2021
Topics include amendments to the Drug Schedules Regulation, Provincial Prescription Management, continuing educations audits, and more.
How to Watch
Along with organizations across Canada, College staff and Board members have been doing our part to reduce the spread of COVID-19 by working remotely. As such, this month’s Board meeting was conducted virtually, via video conference.
A recording of the September Board Meeting is available here:
LAND ACKNOWLEDGEMENT
On behalf of the College of Pharmacists of BC, Board Chair Claire Ishoy, acknowledged and thanked the Sylix Okanagan people on whose unceded traditional territories she chaired the September 24, 2021 meeting. With Board members meeting virtually in different locations across BC, Chair Ishoy also acknowledged that Indigenous Peoples are the traditional stewards of the lands and waters from which each member attended the meeting from.
To the Indigenous peoples of this place we now call British Columbia: We turn our minds to you and to your ancestors. You have kept your unceded homelands strong. We are grateful to live and work here.
Orange Shirt Day Donation
Board Chair, Claire Ishoy, began the meeting by calling attention to Orange Shirt Day and honouring Residential School Survivors, their families and their communities.
Orange Shirt Day, which falls on September 30, is a day for all of us to discuss, recognize and build awareness of the harmful legacy that Canada’s residential school system has left on generations of Indigenous families and communities; and to stand up against racism, bullying and unethical treatment in all facets of Canadian society, from education to health care.
Started by Phyllis Webstad from the Stswecem’c Xgat’tem First Nation, the first Orange Shirt Day took place on September 30, 2013. The date was chosen because it is the time of year during which First Nations children were taken from their homes and put into residential schools. The Orange Shirt refers to the new shirt that Phyllis was given by her grandmother for her first day of school at St. Joseph’s Mission residential school in British Columbia.
In acknowledging this important day, Chair Ishoy announced that the College Board will be making a donation of $500 to the Orange Shirt Society.
The Orange Shirt Society is a non-profit organization based in Williams Lake, BC where Orange Shirt Day began. The society is comprised of both Indigenous and non-Indigenous board members and is focuse on:
- Supporting Indian Residential School Reconciliation
- Creating awareness of the individual, family and community inter-generational impacts of Indian Residential Schools through Orange Shirt Society activities, and
- Creating awareness of the concept of "Every Child Matters"
Lifelong Learning and Accreditation
Colleen Janes, Executive Director of the Canadian Council on Continuing Education in Pharmacy (CCCEP) presented to the Board a brief overview of the origins and mandate of CCCEP. Her presentation focused on the benefits of lifelong learning and the value of accreditation in ensuring learning is of high quality before going on to discuss the strategies envisioned by CCCEP and an exploration of how CCCEP and the College can collaborate on these objectives.

CCCEP is a national organization established to accredit continuing pharmacy education programs intended to be delivered to pharmacy professionals from more than one province or nationally.
CCCEP accreditation is recognized by the pharmacy regulatory authorities in all provinces and territories of Canada. To fulfill its mandate as the national accreditation agency for continuing education in pharmacy, CCCEP:
- Establishes policy and standards for the accreditation of continuing pharmacy education programs;
- Accredits continuing education programs for pharmacists and pharmacy technicians; and
- Accredits program providers to accredit their own continuing pharmacy education programs in accordance with CCCEP standards and guidelines for accreditation.
CCCEP’s Strategic Plan for 2020-2023 includes two objectives relevant to the College:
“Collaborate with regulators to explore the learning needs of pharmacy professionals from a regulatory perspective”
“Consult with pharmacy regulators and professional associations to identify their needs regarding the promotion of the continuing competence of pharmacy professionals and explore partnership opportunities for communicating the value of accredited continuing education and the role of CCCEP in that regard”

Moving forward, CCCEP is looking forward to collaborating with the College in order to drive awareness of lifelong learning and accreditation value among pharmacy professionals; and leverage the importance of developing needs-based lifelong learning programs and accreditation for reputation development with program providers and sponsors.
Excellence: Good, Better, Best!
In August of 2021, the College achieved the CAE Gold certification in Excellence, Innovation and Wellness.
Catherine Neville, Vice President and Lead Client Strategist for Excellence Canada, presented to the Board the significance of this certification, congratulating and recognizing College staff for all of their contributions to this achievement. She then went on to provide a brief overview of Excellence Canada and the Organizational Excellence Standard and provided a review of the College’s progress to date.
In August of 2021, the College achieved the CAE Gold certification in Excellence, Innovation and Wellness.
Based on the Principles and Drivers of Excellence, the Excellence, Innovation and Wellness® Standard is a holistic strategic framework, which helps organizations reduce redundancies, waste and costs while improving productivity and competitiveness. By employing this Standard, organizations can become more effective across all drivers, including Leadership, Planning, Customers, People and Processes.
The Excellence Canada team identified practices that stood out during the assessment process, including:
- Role model in your sector, e.g., agility demonstrated with use of technology and innovative practices to “not miss a beat” when the pandemic hit, where others in your field were struggling
- Common, shared belief in the mission of the College, across all areas
- Focus on Cultural Safety and Humility and equity and inclusion, e.g., Black Lives Matter Working Group, Apology to Indigenous Peoples, Pledge to Cultural Safety and Humility
- Intentional “People first” culture where people feel cared about and supported
- Cross functional working committees that are improving, innovating, and communicating
- All staff meetings where information is shared and people are celebrated, e.g., Daily Dose recognition
- Board surveys after each meeting and Governance Committee reviews results
- Transparency at both the Board and College levels, e.g., public board meetings available online
The College of Pharmacists of BC has satisfied the requirements and scoring for the Excellence, Innovation and Wellness® Standard at the Gold level and is now eligible to receive CAE Gold recognition at the Canada Awards for Excellence ceremony in the fall of 2021.
Patient Voices Network – A Platform for Partnerships
Engagement Leaders Jamie Brown and Charmaine Niebergall with the BC Patient Safety and Quality Council presented to the Board some of their learnings as the administrator of the Patient Voices Network.
For over 11 years, Patient Voices Network (PVN) has served as a platform for partnerships between health care and patients, families, and caregivers to learn from each other and strive to improve health care for all British Columbians.
Quality Assurance Committee: Continuing Education Audits
Allen Wu, Chair of the Quality Assurance Committee provided the Board with an overview of the Continuing Education (CE) Audit process and the 2020 results.
As part of the Quality Assurance Program, set out in the College’s Health Professions Act Bylaws, CE is mandatory for all registered pharmacy professionals as one of the requirements for registration renewal.
The College ensures pharmacy professionals meet the CE requirements through the Professional Development and Assessment Program (PDAP), which requires CE to be documented and submitted through at least 6 learning records annually. Each pharmacy professional must complete a minimum of 15 hours of CE, of which a minimum of 5 hours must be accredited CE.
The College’s Quality Assurance Committee (QAC) provides guidance and recommendations to the College Board on minimum CE requirements, standards, and processes for monitoring and auditing CE submissions for compliance with the requirements. CE Audits play an important role in ensuring registrants are completing CE requirements, help identify opportunities to apply the learning to their practice and helps the QAC identify opportunities for improvement of the process.
The QAC consulted with a statistician for recommendations regarding the CE Audit process and criteria.
The statistician recommended an audit of 400 pharmacy professionals each year. Of the 400 registrants that were audited, 365 were randomly selected from the 2020 registration deadline renewal pool, and 35 were automatically audited as part of the process of re-instating to practice. Of the 365 registrants that were randomly selected and audited, 303 were pharmacists and 62 were pharmacy technicians.
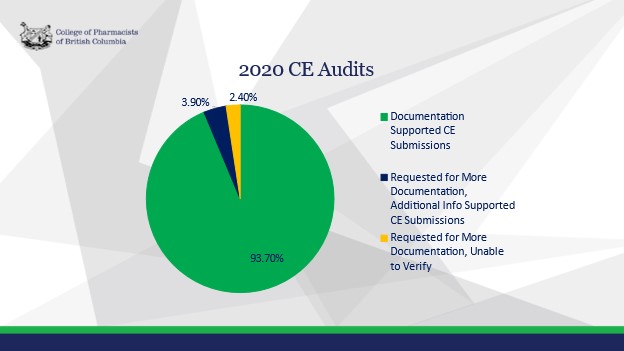
All registrants who were audited received correspondence regarding the audit and outcome. Overall, the 2020 CE Audit results identified the following recommendations for all registrants when submitting compliant Learning Records:
- Attach supporting documentation from the CE activity provider that verifies the learning is accredited and contains all the required information including:
- Certificate of completion
- Learning summaries/CE Profiles (from CE providers) if they contain all the required information
- Ensure that supporting documentation is consistent with the Learning Record, especially in the following areas:
- Registrant name
- Learning activity title
- Date of completion is within the CE renewal cycle
- Hours of learning
- Avoid duplicate entries
- Retain all original supporting documentation for at least 2 years from the registration renewal deadline to support the submission
The information gathered by the CE Audits enables the identification of trends in non-compliance that can be used to support registrants in submitting compliant Learning Records, and to help improve tools and communications on the website and portal.
The Quality Assurance department will continue to collect and evaluate data collected through CE Audits along with information gathered from registrant feedback (e.g., emails, PDAP Portal and PDAP Mobile Survey Feedback) to identify opportunities for future improvement of PDAP.
Registrar Search Committee Update
Bob Nakagawa, Registrar/CEO of the College has indicated that he will be retiring in the Fall of 2021. As such, on April 30, 2021, the Board approved the establishment of a Registrar/CEO search committee to oversee the process of recruitment and selection of a suitable candidate. This committee will provide a recommendation to the Board for a new Registrar/CEO.
The Board appointed the following Board members to the Registrar Search Committee:
- Board Chair, Claire Ishoy
- Board Vice-Chair, Steven Hopp
- Board Member, Andrea Silver
- Public Board Member, Katie Skelton
- Public Board Member, Justin Thind
The Committee has met every two weeks throughout the summer to complete the following activities:
- Selection of Odgers Berndtson as the search firm to assist the Board;
- Develop and finalize the candidate profile;
- Approved the methodology of the search firm;
- Review candidates;
- Generate long list of applicants; and
- Discuss compensation and benefits.
To date, the Committee has received a number of applications from high quality candidates who remain confidential at this time. The long list of candidates was presented to the Board on September 16th with the first round of interviews held on September 22 and 23, 2021.
Provincial Prescription Management – A Ministry of Health Project
Taryn Drlik, PharmaNet Clinical Lead at the Ministry of Health, introduced the Board to the Provincial Prescription Management project (PPM), a broad-scale transition to electronic management of prescriptions in PharmaNet, including e-prescribing.
While the concept of e-prescribing is not new, the Ministry is approaching it with a renewed vision for interdisciplinary care. A cornerstone of PPM is enhanced information-sharing across care providers and care settings in real time. All members of a patient’s care team will be equipped with a single comprehensive medication profile, improving prescribing practices, patient safety, and patient outcomes. This renewed approach to e-prescribing was born in response to feedback received from stakeholders across the health sector. More than 70% of respondents identified the need to expedite implementation of a digital prescription management solution that would integrate PharmaNet with EMRs for seamless documentation of a patient’s prescription information.
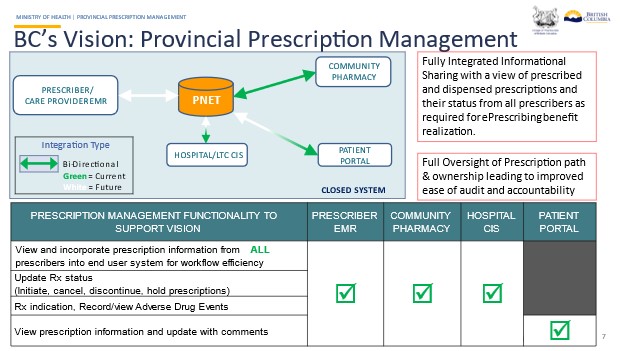
The initial launch of PPM will include the integration of community clinical end user systems (EMRs, pharmacy systems) and in a later phase, integration with health authority clinical information systems to support transitions in care between acute and community.
The College recognises the overarching value and key benefits that PPM can deliver and will be supporting this important initiative through helping to share Ministry of Health PPM updates with pharmacy professionals. Stay tuned for more detailed project information to be broadcast in October.
For specific inquiries about the PPM project, please contact:
Taryn Drlik, Clinical Lead PharmaNet [email protected]
Libni Pardo, Project Manager, PPM [email protected]
Legislation Review Committee: Drug Schedules Regulation – Amendments
The Board approved amendments to the Drug Schedules Regulation (DSR) under the Pharmacy Operations and Drug Scheduling Act (PODSA) to improve alignment with the National Drug Schedules (NDS) and the Prescription Drug List made under the Food and Drugs Act (Canada), and to update the definition of Schedule IA (Triplicate/Duplicate Prescription Program).
The approved amendments to the DSR align with changes made to the NDS and the Prescription Drug List, in the interest of public safety.
As directed by the Board in September 2020, amendments were approved to help improve the DSR’s alignment with NAPRA’s natural health product (NHP) policy. In late 2019, NAPRA announced that it would begin removing NHPs from the NDS in a step-wise manner, as NHPs are federally regulated. NHP’s became subject to federal regulation in 2004 under the Natural Health Products Regulation, under the Food and Drugs Act.
As of 2024, NAPRA will consider all products with a Natural Health Product Number or Drug Identification Number-Homeopathic Medicine issued from Health Canada to be outside of its scope. For more information, see the “Background on Update to NAPRA NHP Policy,” and the September 2020 Board Highlights.
Lastly, the Board approved an amendment to the definition of “Schedule IA (Duplicate/Triplicate Prescription Program)”. The current definition refers to the Pharmacist, Pharmacy Operations and Drug Scheduling Act, which is no longer in existence. The amended definition refers to the Pharmacy Operations and Drug Scheduling Act.
These amendments have been filed with the Minister of Health for a period of 60 days, after which they will be deposited with the Registrar under the Regulations Act. These amendments are expected to come into force in November 2021.
Housekeeping Amendments to Professional Practice Policy-66: Opioid Agonist Treatment
The Board approved amendments to Professional Practice Policy-66: Opioid Agonist Treatment (PPP-66), related to the upcoming deadline to complete the Opioid Agonist Treatment Compliance and Management Program (OAT-CAMPP).
The deadline to complete OAT-CAMPP is September 30, 2021, which means that as of October 1, 2021, all pharmacy managers, staff pharmacists, relief pharmacists and pharmacy technicians employed in a community pharmacy that provides pharmacy services related to opioid agonist treatment (OAT) must complete any applicable component(s) of OAT-CAMPP in order to fulfill the College’s Opioid Agonist Treatment training requirements as outlined in PPP-66.
Previously, the College’s Methadone Maintenance Treatment (MMT) training program was the only training program required, however as of October 1, 2021, it will no longer meet the requirements in PPP-66 and thus will be removed from the College’s website.
In addition, as of October 1, 2021, the Ministry of Health’s PharmaCare program will also require that every pharmacy employed in a pharmacy that provides OAT services must complete the OAT-CAMPP training.
Both the Ministry of Health and the College have communicated the deadline to registrants.
With September 30, 2021 soon approaching, the approved amendments will repeal soon-to-be outdated provisions within PPP-66 related to the transition period. Consequential amendments to the PPP-66 Methadone Maintenance Policy Guide were also approved. These amendments will come into force on October 1, 2021 at which point the existing version of this policy will be replaced with the amended one on the College’s website.
 Share
Share


