Board highlights - September 18, 2020
Topics include Amendments to the Standards for Drug Administration by Injection and Intranasal Route; the PRP Annual Report; the Removal of Natural Health Products from the DSR and more.
How to Watch
Along with organizations across Canada, College staff and Board members have been doing our part to reduce the spread of COVID-19 by working remotely. As such, this month’s Board meeting was conducted virtually, via video conference.
A recording of the September Board Meeting is available here:
Drug Administration Committee: Proposed Amendments to the HPA Drug Administration by Injection and Intransal Route Standards, Limits, and Conditions
The Board directed the Registrar to engage with the Ministry of Health to move proposed amendments to the Drug Administration by Injection and Intranasal Route Standards, Limits and Conditions forward. Consideration will be given to how the amendments support the response to COVID-19 and help ensure that the public can receive the health services they need in a safe manner.
In light of the aim to engage with the Ministry of Health, the Board deferred considering the acceptance of the proposed amendments, to their November 2020 meeting.
Currently, the College’s Drug Administration by Injection and Intranasal Route Standards, Limits and Conditions (“Standards, Limits and Conditions”) only permits a pharmacist to administer a drug for the purpose of immunization.
At its February 2019 meeting, based on recommendations from the Drug Administration Committee, the Board directed the Registrar to remove certain current restrictions on pharmacist injection and intranasal administration of medications.
In the spring of 2019, the College began work with the Professional Regulation and Oversight Branch of the Ministry of Health to establish the Safe Drug Administration by Pharmacists Working Group. This Working Group, comprised of representatives from the College, the Ministry of Health, and regulatory colleges of health professions with prescribing authority, identify and discuss the impacts of removing the restrictions on pharmacist injection and intranasal administration of medications.
On August 14, 2020, the Drug Administration Committee met and approved proposed amendments to the Standards, Limits and Conditions, and recommended the changes to the Board for consideration.
The proposed amendments were informed by cross-jurisdictional research, review by internal College and external stakeholders, as well as clarification needs. The key proposed amendments include:
- Allowing administration of Schedule I and II drugs with the exception of Schedule IA
- Prohibiting injection of cosmetic drugs and substances
- Maintaining existing age limits (prohibits the administration of injections to children under 5 years old and the administration of a drug by intranasal route to a child under 2 years old)
It is not yet known when the proposed changes will take effect. As directed by the Board, the Registrar will engage with the Ministry of Health to move the amendments forward.
The College will communicate with registrants and the public when the amendments will be in force.
COVID-19 Budget Review and Fee Increase Consideration
The Board directed the Registrar to implement the following annual fee increases as stated in the 2020-21 budget:
- 5.25% increase for pharmacists and pharmacy technicians, effective November 2020
- 5.5% increase for pharmacies, effective April 2021
The Board approved the 2020/21 budget at its February Board meeting, which included these planned fee increases. However, with the onset of the novel coronavirus pandemic in March, a decision was made at the April Board meeting to review the impact of the COVID19 pandemic before proceeding with the planned fee increases that had been included in the approved budget.
Just like many other businesses and families, COVID-19 has had a profound impact on the College’s financial bottom line. As a result of the important safety measures implemented in BC and across Canada, the College has experienced a 4% decline in its anticipated revenue for this fiscal year.
Most notably, with many pharmacy and pharmacy technician students unable to complete necessary exams, there has been a significant decline in anticipated new licence registrations and the additional fees that come with those.
The College has worked hard to find savings to mitigate the impact of the pandemic, reducing expenses by 16%. However, after reviewing the impact of adjusted operations due to COVID-19, the College has determined that it needs to move forward with its planned fee increase. The increase makes sure the College can continue its essential operations while continuing to take action on important strategic initiatives needed for public safety, and maintaining a fiscally responsible reserve balance.
As projected in June 2020, the College’s financial situation shows sufficient funding and reserve balances for this year and next year. However, it is during the 2022/23 and 2023/24 fiscal years that the closing reserve balances become a serious concern due to continued deficits.
Because it generally takes almost three years for the revenue generated by fees to be realized in the College’s revenue, these fee increases must be implemented this year in order to ensure sufficient funding during the 2022/23 and 2023/24 fiscal years.
The Board approved amendments to the Health Professions Act Bylaws Schedule D – Fee Schedule for filing with the Ministry of Health.
Amendments to the Fee Schedule in the Pharmacy Operations and Drug Scheduling Act Bylaws have been posted for public comment on the College website. Once the 90-day public posting period is completed, the PODSA Fee Schedule will be brought to the Board for filing approval.
Update from the Pharmacy Examining Board of Canada
Gabriella Wong, a member of the Board of Directors of the Pharmacy Examining Board of Canada (PEBC), provided the College Board with an update regarding the implications of COVID-19 on the PEBC assessments and examinations.
The PEBC is the national certification body for the pharmacy profession in Canada. PEBC is a non-profit organization with more than 50 years of experience in assessing the qualifications and competence of candidates for licensing by pharmacy provincial regulatory authorities.
The purpose of the PEBC is to assess qualifications for pharmacists and pharmacy technicians on behalf of participating provincial regulatory authorities. The PEBC Board evaluates qualifications, develops and administers examinations including a national Qualifying Examination, and issues Certificates of Qualification.
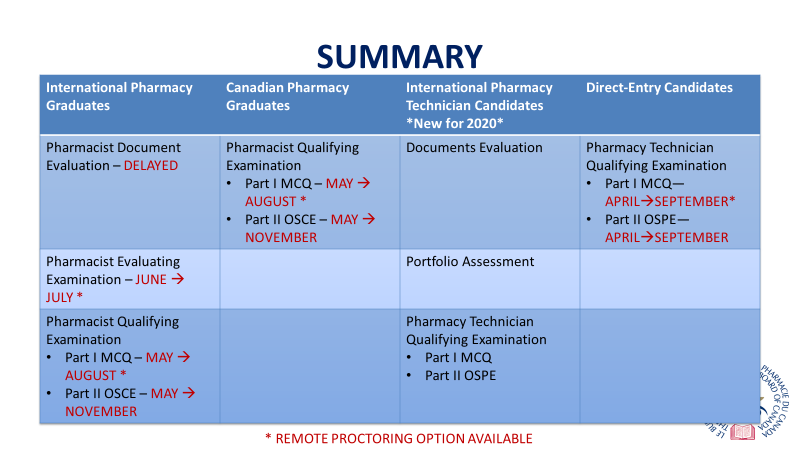
The COVID-19 pandemic has resulted in changes to the administration of PEBC exams.
Pharmacists and pharmacy technicians are required to complete Qualifying Examinations (QEs) designed to assess entry-level competencies required for safe and effective practice. The QE examines the ability of candidates to apply their knowledge, skills and abilities to solve practice-based problems and meet patients’ needs.
The QE consists of a written multiple choice exam (MCQ), and an objective, structured performance exam (OSPE) for pharmacy technicians and an objective, structured clinical exam (OSCE) for pharmacists.
Before COVID-19, QEs were administered twice per year, once in the spring and once in the fall. The MCQ was administered across 17 Prometric Testing Centres, while the OSPE and OSCE was administered at sites across the country.
In March 2020, at the onset of COVID-19 related restrictions across the country, PEBC cancelled the QEs scheduled in the spring.. Additionally, the PEBC Board approved the use of remote proctoring as an additional examination delivery modality for MCQ exams.
Influenza Season and COVID… Now What?
Joanne Archer, Education and Practice Coordinator for the Provincial Infection Control Network of British Columbia (PICNET), provided the Board with updated guidance on the challenges posed by COVID-19 on Immunization administration during the upcoming influenza season.
The Provincial Infection Control Network of British Columbia (PICNet) is a provincial program of the Provincial Health Services Authority that works to reduce healthcare-associated infections in BC healthcare facilities.

In her presentation, Ms. Archer outlined the following Immunization challenges posed by COVID-19:
- Need for measures to avoid transmission of COVID-19 to staff
- Access to sufficient supplies of PPE for vaccinators and other staff
- Access to or suitability of usual venues for immunization administration
- Public fear of exposure to COVID-19 while accessing immunization services
- Potentially increased demand for influenza vaccine starting early in the campaign, as seen in the Southern hemisphere.
In addressing these challenges, there are a number of opportunities available for healthcare providers with regard to updating their immunization protocols. These include:
- Immunization appointments provide an avenue to assess the needs of the client for education and advice on immunizations in general, influenza and COVID-19 in particular
- Develop and practice approaches that may be used for the anticipated COVID-19 massive immunization program
- Identify diverse needs of groups based on: access to services, vulnerability, ethnicity/culture and other socioeconomic factors
Ms. Archer went on to identify the following considerations for Influenza Vaccine providers as we approach the upcoming 2020 flu season:
- Extend immunization availability hours to avoid crowding
- Use an appointment system
- Designate a specific staff member(s) for immunizations to avoid disrupting the dispensing of medications
- Outdoor or drive through clinic if appropriate
- Mobile clinic that can visit a neighborhood or small remote community
- Funnel clients in a one way direction that also maintains physical distancing while waiting
For more information see the BCCDC’s Guidance for Influenza Vaccine Delivery in the Presence of COVID-19.
Practice Review Committee: Practice Review Program Annual Report
Tracey Hagkull, Chair of the Practice Review Committee and James Van, Community Pharmacy Compliance Officer, presented Practice Review Program Annual Report for the 2019-2020 fiscal year to the Board.
The Practice Review Program (PRP) is an in-person review of a pharmacy professional's practice and the pharmacy where they work. The program aims to protect public safety by improving compliance with College Bylaws and Professional Practice Policies and ensuring consistent delivery of pharmacy services across BC.

The Practice Review Program is split into two components; the Pharmacy Review and the Pharmacy Professionals Review. The Pharmacy Review focuses on the legislated physical requirements of a pharmacy and the responsibilities of a pharmacy manager. The Pharmacy Professionals Review focuses on areas identified and approved by the Board as having the greatest impact on patient safety including patient identification verification, profile check, counselling, documentation, product distribution and collaboration.
For the 2019-2020 fiscal year, 279 community and 13 hospital pharmacy sites were reviewed. In addition, 666 community pharmacists, 77 community pharmacy technicians, 241 hospital pharmacists, and 200 hospital pharmacy technicians were reviewed.
Overall results of the practice review process have been positive, with average compliance percentages of 93% for community and 87% for hospital pharmacies before any corrective action items were completed.
The top areas of non-compliance are listed below. For further insights into each of the areas of non-compliance, see the Practice Review Program Annual Report. The College will be sharing learnings from the top areas of non-compliance through PRP Insights ReadLinks articles – stay tuned.
Pharmacies
Community Pharmacies
| 2019-2020 |
|---|
| 1. Prescriptions |
| 2. Inventory Management |
| 3. Pharmacy Manager Responsiblities |
| 4. Equipment and References |
| 5. Security |
Hospital Pharmacies
| 2019-2020 |
|---|
| 1. Sterile Compounding |
| 2. Inventory Management - Nursing Unit |
| 3. Ambulatory Service |
| 4. Pharmacy Manager's Responsibilities |
| 5. Equipment and References |
Pharmacists
Community Pharmacists
| 2019-2020 |
|---|
| 1. Counselling |
| 2. Documentation |
| 3. Patient Identification Verification |
| 4. PharmaNet Profile Check |
Hospital Pharmacists
| 2019-2020 |
|---|
| 1. Counselling |
| 2. Documentation |
| 3. Profile Check |
| 4. Patient Identification Verfication |
Pharmacy Technicians
Community Pharmacy Technicians
| 2019-2020 |
|---|
| 1. Documentation |
| 2. Product Distribution |
| 3. Collaboration |
| 4. Patient Identification Verification |
Hospital Pharmacy Technicians
| 2019-2020 |
|---|
| 1. Documentation |
| 2. Patient Identification Verification |
| 3. Collaboration |
| 4. Product Distribution |
Satisfaction Survey
Once a practice review is completed, pharmacy professionals are invited to participate in an optional and anonymous online survey which measures the agreement rating of respondents to their PRP experience and its processes.
Overall, the feedback received was overwhelmingly positive, with an average agreement rating of 90.74%.
Removal of Natural Health Products from the Drug Schedules Regulation
The Board directed the Registrar to remove natural health products (NHPs) from the Drug Schedules Regulation in a step-wise manner to align with the removal of natural health products from the National Association of Pharmacy Regulatory Authorities’ (NAPRA’s) National Drug Schedules.
Prior to 2004, NHPs were sold as either food or drugs under the Food and Drugs Act because there was no other category under which to classify them. In 2004, NHPs became subject to federal regulation under the Natural Health Products Regulation (NHPR). Under the NHPR, Health Canada requires evidence of the efficacy and safety of an NHP prior to issuing a license, and there are different licensing pathways for NHPs that make modern health claims, and those that are used as traditional medicines.
NAPRA does not typically schedule NHPs on the National Drug Schedules. However, some products listed on the National Drug Schedules became reclassified as NHPs after the NHPR came into effect. This created a scheduling discrepancy, as only this subset of NHPs were scheduled on the National Drug Schedules. NAPRA agreed to keep the reclassified NHPs on the National Drug Schedules on an interim basis.
In late 2019, NAPRA announced that it would begin removing NHPs from its National Drug Schedules in a step-wise manner.
As of 2022, NAPRA will consider all products with a Natural Product Number (NPN) or Drug Identification Number-Homeopathic Medicine (DIN-HM) issued from Health Canada to be outside of its scope. For more information, see the “Background on Update to NAPRA NHP Policy.”
BC’s Drug Schedule Regulation closely aligns with the National Drug Schedules, and also only contains a small subset of NHPs. Removing NHPs from the Drug Schedule Regulation is consistent with NAPRA’s policy decision, and with the approaches of other Canadian jurisdictions.
The College will collaborate with stakeholders as it moves forward with implementing the Board direction, , focusing on the removal of Schedule I and II NHPs in 2022.
Implementing of the National Association of Pharmacy Regulatory Authorities' Model Standards for Pharmacy Compounding
Compounding, in respect to a drug, is defined as the mixing together of one or more other ingredients. Healthcare professionals who provide compounding related services and products to patients, must be able to demonstrate that a patient-healthcare professional relationship exists.
Evolving practice and increased awareness of the inherent dangers of compounding sterile preparations for the health of both patients and compounding personnel, led the National Association of Pharmacy Regulatory Authorities (NAPRA) to develop a suite of new model standards for pharmacy compounding. These model standards will set national standards for pharmacy compounding and are expected to be adopted by pharmacy regulatory authorities across Canada.
The Sterile Model Standards and Non-Sterile Model Standards will come into effect in each province/territory once they have been adopted by the respective provincial/territorial pharmacy regulatory authorities.
In April 2017, the Board approved a four-year, phased implementation plan to adopt both the Sterile Model Standards.
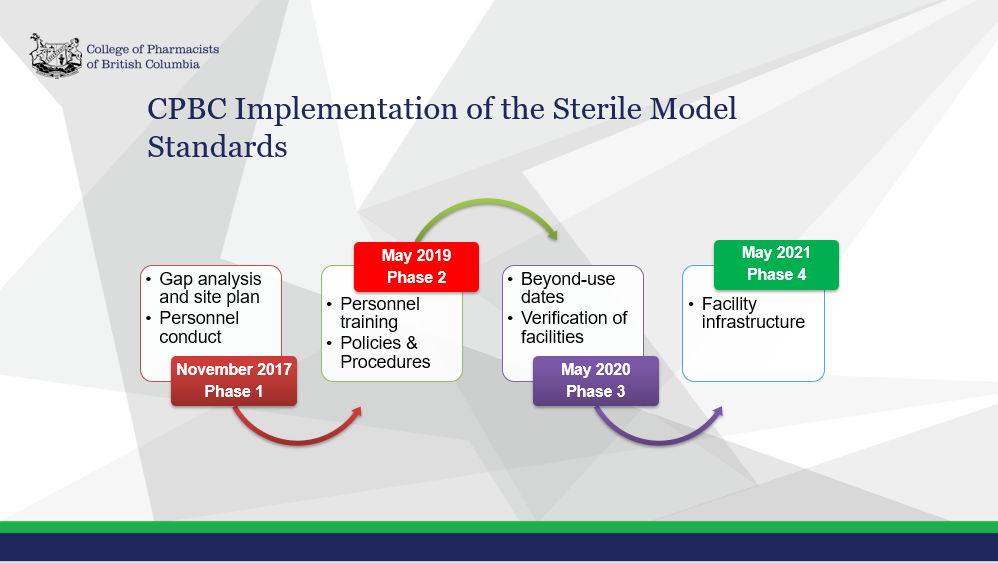
In June 2020, College staff surveyed pharmacies to assess the status of their compliance with the Sterile Model Standards. In general, the results from the survey indicate that hospital sites and community pharmacies are progressing towards compliance with the Sterile Model Standards. However, the survey results also indicated that the majority (84%) of sites will not be compliant with the Sterile Model Standards by May 2021.
The impact of the COVID-19 pandemic is a key reason why pharmacies are unable to meet the original May 2021 deadline. With the unforeseen onset of COVID-19, pharmacies had to focus on issues related to the pandemic.
This one-time only extension of the deadline from May 2021 to July 2022 provides hospital and community pharmacies additional time to implement the Sterile Model Standards, in recognition of the COVID-19 pandemic. The majority of pharmacies surveyed are expecting to by compliant by this extension deadline.
 Share
Share