BOARD HIGHLIGHTS - NOVEMBER 18, 2016
Highlights from this meeting include election results for the College Board Chair and Vice-Chair, new practice standards, Practice Review Program for Hospital Practice, the College's new strategic plan, and the results of the Certified Pharmacist Prescriber engagement.
You can re-watch all the November Board meeting presentations through the College's Periscope Channel.
Board Chair and Vice-Chair Election Results
The College Board elections for Chair and Vice-Chair took place at the November meeting.
Chair: Anar Dossa, District 6 - Urban Hospitals representative, was elected Board Chair.
Vice-Chair: Mona Kwong, District 1 - Metropolitan Vancouver representative, was elected Vice-Chair.
Deputy Registrar Appointment
The College Board approved the appointment of David Pavan as The Deputy Registrar of the College of Pharmacists of British Columbia in accordance with the Health Professions Act Bylaws Section 22(2).
David comes to us with over 20 years of pharmacy management and leadership experience in both rural and urban settings. Most recently, he served as BC Pharmacy Operations Specialist for Shoppers Drug Mart. David has also been an active Board Member with the BC Pharmacy Association, serving as their President in 2015.
GOVERNANCE COMMITTEE UPDATE
Board Member and Chair of the Governance Committee Norm Embree provided an update on the Governance Committee’s activities since the last Board Meeting.
The Board approved the motion allowing the Governance Committee to continue to move forward with the second phase of an organizational review which will focus on the Board and external stakeholders. The organizational review was approved by the Board at the June 2016 Board Meeting and the initial phase included an internal staff review.
Governance Committee: Elected Board member terms of office
The College Board directed the Registrar to pursue Bylaw amendments to change the term of office for elected Board Members from two years to three years, and from a maximum of 3 consecutive terms to a maximum of 2.
Evidence for the Benefit of an Advanced Scope of Pharmacy Practice – The Alberta Experience
Dr. Ross T. Tsuyuki, Professor of Medicine at The University of Alberta, and Director of The EPICORE Centre presented new research to the Board on the impact advanced scope of pharmacy practice has for patient outcomes in Canada and globally.
Dr. Tsuyuki emphasized the opportunity pharmacists have in improving the accessibility of timely care – a top priority identified in the 2015 Healthcare in Canada Survey. Pharmacists generally see patients (especially those with chronic diseases) more frequently than any other healthcare professional which makes them makes them highly accessible. And pharmacists are highly trusted by the public. For this reason, the range of healthcare services included within a pharmacists’ scope of practice has been continuing to expand to better meet the needs of patients.
The presentation highlighted the results of his recent clinical trial and the evidence on the impact of pharmacist prescribing and laboratory testing in patients with, or at risk for, cardiovascular diseases.
Evidence that pharmacist prescribing intervention reduced patients' risk for major cardiovascular events by 21%. #CPBCBoard pic.twitter.com/HWJt2sM8qW
— BC Pharmacists (@BCPharmacists) November 18, 2016
Legislation Review Committee:
Drug Schedules Regulation
The College Board approved amendments to the Drug Schedules Regulation, B.C. Reg. 9/98, subject to filing with the Minister, to align with recommendations that the National Drug Scheduling Advisory Committee made in March 2016.
The amendments include:
| Drug | Existing | New |
|---|---|---|
|
ibuprofen or its salts, when sold in a modified-release oral dosage form that provides 600 mg or less per dosage unit (indicated for relief of rheumatoid arthritis, and osteoarthritis). |
Schedule I |
Schedule III |
|
esomeprazole or its salts, when sold for the 14-day treatment for frequent heartburn at a daily dose of 20 mg, in package sizes of no more than 280 mg of esomeprazole |
Schedule I |
Schedule II |
|
fluticasone propionate, when sold for the treatment of allergic rhinitis in a nasal spray that delivers 50 mcg/spray for those 18 years of age and older, in package sizes containing no more than 120 metered sprays |
Schedule I |
Schedule III |
| fluticasone propionate, when sold for the treatment of allergic rhinitis in a nasal spray that delivers 50 mcg/spray for those 18 years of age and older, in package sizes containing more than 120 metered sprays | Schedule I |
Schedule II |
This resolution has been submitted for final approval by the Ministry of Health. The College will notify registrants when the amendments come into force – 60 days from when the tagged schedule is deposited with the Registrar of Regulations.
HPA Bylaws – Application Committee
The College Board approved proposed amendments to Health Professions Act Bylaws for public posting – they establish a new Application Committee that will be needed when new amendments to the Pharmacy Operations and Drug Scheduling Act (PODSA) come into force.
PODSA amendments – which the College is working together with the Ministry of Health to implement – give the College the authority to identify pharmacy owners. As part of this change, an Application Committee is required to review pharmacy licence applications that do not meet the requirements in PODSA.
The College is asking for your feedback on the proposed HPA Bylaws establishing the Application Committee. The amended HPA Bylaws will be publicly posted for a 90 day period, after which College staff will review the comments received and finalize the Bylaws for filing.
The final Bylaws and draft Terms of Reference for the Application Committee are expected to be brought forward for the Board’s consideration at the April 2017 Board meeting.
See the proposed HPA amendments establishing an Application Committee and provide your feedback.
HPA Standards of Practice: Parts 1, 2 and 3, and new PPP-75
The College Board approved amendments to the Health Professions Act (HPA) – Bylaws, Schedule F, Parts 1, 2, and 3 that create minimum standards for registrants regarding the preparation of a prescription product, final product check, and patient identification for filing with the Ministry of Health.
The Board also approved Professional Practice Policy #75: Patient Identification which establishes patient identification requirements where there is no face-to-face interaction between a patient and a registrant.
The College would like to thank all the College Committee Members who contributed input throughout the development of these new standards.
The new standards will be incorporated into the College’s Practice Review Program and will ensure there are clear requirements for preparation of a prescription product, final product check, and patient identification.
The amended bylaws will be filed with the Minister of Health and will come into effect 60 days after filing. The College will notify registrants when the new standards come into effect.
Jeremy Walden, Chair of the legislative committee will now present to #CPBCBoard pic.twitter.com/ZePZRuNeBJ
— BC Pharmacists (@BCPharmacists) November 18, 2016
Certified Pharmacist Prescriber
Report on Stakeholder Engagement
After receiving Board approval on the Certified Pharmacist Prescriber Draft Framework in November 2015, the College engaged with pharmacy professionals, other prescribers and patients to solicit feedback.
The consultation period ran from February 2016 to August 2016 and included 15 different workshops and stakeholder meetings with over 25 different groups and organizations as well as an online survey which received more than 11,400 comments from over 1,500 respondents.
CPP Engagement; 15 workshops, meeting w/ 200+ ppl, 1500+ survey responses, and 11,400+ comments! #CPBCBoard pic.twitter.com/5bjFqcNK7K
— BC Pharmacists (@BCPharmacists) November 18, 2016
The Engagement Report consolidates all the feedback received through the pharmacist prescribing engagement under four key themes:
- Confidence in pharmacists prescribing
- Collaboration and communication
- Improving patient care
- Support for the initiative.
Overall, stakeholder groups were quite divided in their level of confidence in pharmacists prescribing as well as their support for the initiative. Feedback indicated overwhelming support from pharmacists and pharmacy technicians, but strong resistance from other prescribers, while the public was divided.
The greatest convergence across stakeholder groups surrounded the opportunity pharmacist prescribing could have in providing greater access to care, especially for minor ailments, emergency situations, continuity of care and for patients without a primary care provider. Feedback from pharmacists and other prescribers also highlighted that pharmacist prescribing might work best in interdisciplinary team-based settings where access to more patient information and lab test results, and having a physician or nurse practitioner available to provide a diagnosis, provided respondents with greater confidence in pharmacist prescribing.
Learn more about the results of this engagement through the Certified Pharmacist Prescriber Engagement Report.
Five Board Members met to review the results of the pharmacist prescribing engagement. After much consideration they recommended to the full Board that the scope of the Draft Framework and the proposal to the Minister of Health be narrowed to pharmacist prescribing within a collaborative practice setting.
Collaborative practice settings for pharmacist prescribing would include interdisciplinary team-based settings where physicians and nurse practitioners continue to be responsible for the diagnosis; and where access to health records and diagnostics, including lab tests, would also be required. Certified Pharmacist Prescribers would also be restricted from dispensing medications they prescribed for a patient.
Practice Review Program: Hospital Practice Implementation
The College Board approved the implementation of the second phase of the Practice Review Program: Hospital Practice. The motion was brought forward by the Practice Review Committee and presented to the Board by Committee Chair Michael Ortynsky.
Since May 2015, the College has conducted multiple consultation sessions with hospital pharmacy stakeholders, as well as a Joint Application Design session with the PRP team and subject matter expert consultants to provide input on the design of the hospital practice reviews.
Feedback from these sessions informed key pieces in the development of the Practice Review Program for Hospital Practice including drafting Patient Identification Verification and Prescription Product Preparation/Final Check standards to support the PRP focus areas.
Mike Ortynsky, Chair of the PRC, will now be presenting PRP: Hospital Pharmacy Practice implementation. #CPBCBoard pic.twitter.com/SbJxpUDRyu
— BC Pharmacists (@BCPharmacists) November 18, 2016
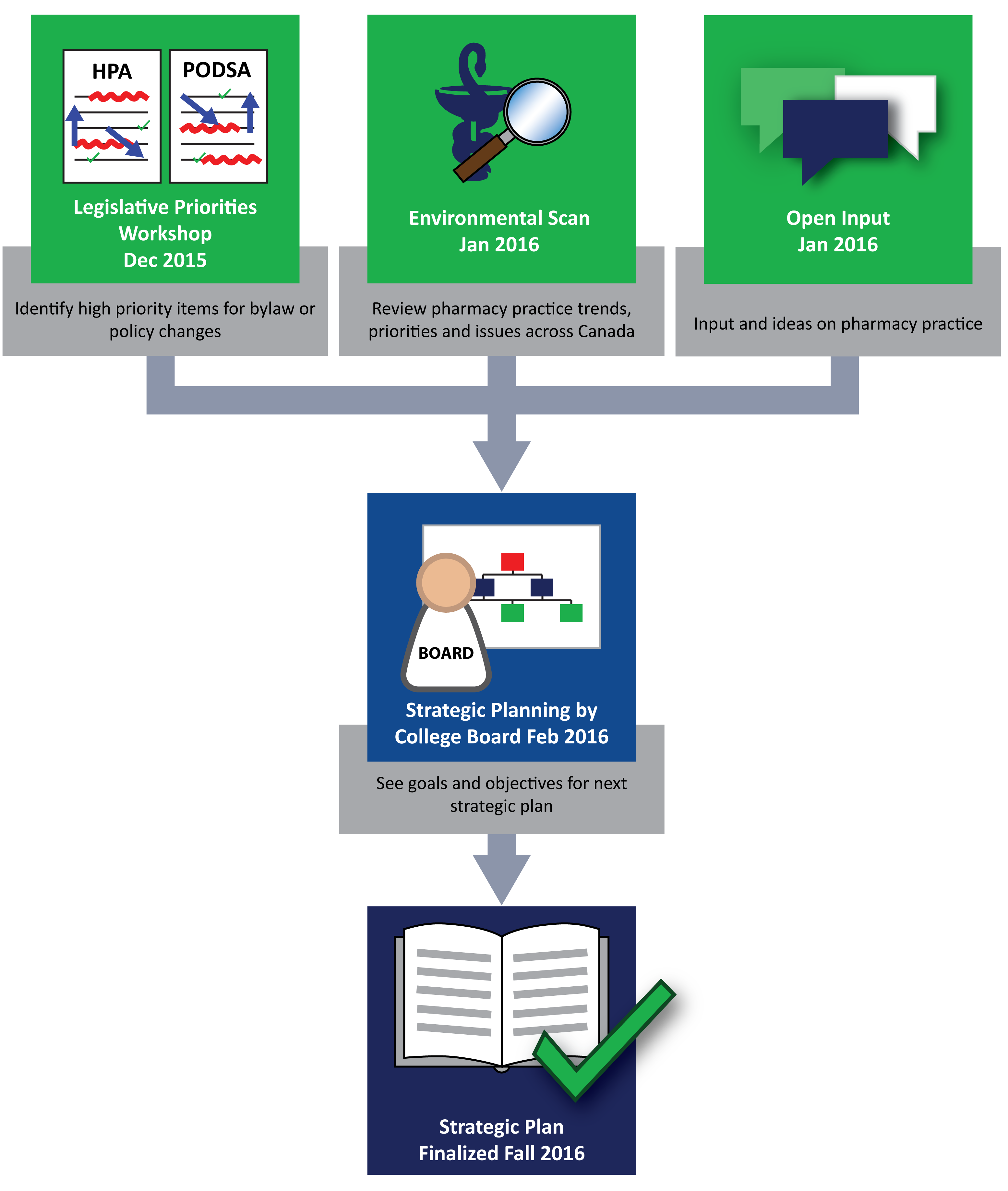
Strategic Plan
The College Board approved the high level Draft Strategic Plan for 2017/18 -2019/20. The College will now build a detailed 3-year Strategic Plan and corresponding budget for approval at the February 2017 Board Meeting.
To set the priorities for the next three years the Board reviewed feedback from stakeholders through a ThoughtExchange which included over 5,000 thoughts from 1300 participants. An environmental scan was also shared with the Board in addition to the results of a legislative priorities workshop that identified high priority areas for bylaw or policy changes. The Board also considered staffing and budget implications.
The input gathered was used during the Board Strategic Planning Session to help identify four strategic goals that will enable the College to continue to achieve its Mission over the next three years.
Goal 1: Legislative Standards & Modernization
- The College will work to modernize the legislative requirements under the Pharmacy Operations and Drug Scheduling Act (PODSA) and the Health Professions Act (HPA), to ensure they are clear, consistent and enforceable.
Goal 2: Professional Excellence
- The College will ensure that the practice of pharmacy meets or exceeds the standards set out to protect the public and maintain their trust.
Goal 3: Drug Therapy Access and Monitoring
- The College will explore avenues that enhance the ability of pharmacy professionals to maximize the public’s access to safe, high quality drug therapy.
Goal 4: Organizational Excellence
- The College will ensure that the efficiency and effectiveness of its foundational business processes and technological supports are upgraded to meet the ongoing needs of registrants, pharmacy owners and directors, staff and the public. It will also ensure that College governance and staffing are well organized and provided at the appropriate level.
 Share
Share